iSimulate - EMS World Innovation Award Winners
Oct 23, 2020
EMS World just announced the recipients of the 2020 EMS World Innovation Awards. The award program recognises the industry’s most pioneering products of the year that were on full display at EMS World Expo 2020.
An independent panel of judges consisting of EMS World editorial advisory board members evaluated each entry on its innovation, features, and applicability to EMS delivery. Products must be new or have undergone significant design changes after August 2019. From a pool of finalists, judges met with company representatives for demonstrations of each product, to learn about its application and determine whether it is worthy of this esteemed honour.
This year, the 3B Scientific Group with iSimulate had the honour of receiving an award for the newly upgraded iSimulate REALITi360 Build 10 Simulation Ecosystem.
The iSimulate REALITi360 Build 10 Ecosystem for patient monitor simulation was awarded thanks to its robust new features:
Upgrades and enhancements include a new optional remote module to control the monitor, chart, and camera remotely over the internet; optional ZOLL EMV+ Ventilator premium screen; slider control option to adjust vital sign parameters; ability to manually send a 12-lead printout from the control; Bluetooth-connected audio; preview toggle to the scenario story editor; additional language support; and much more.

Wilmington, Delaware – Avkin creates, manufactures, and sells technologically advanced wearable simulators to the healthcare education market, releasing the ONLY automated, wearable birthing simulator in January of 2022. Amy Cowperthwait, Founder, and CEO of Avkin has made it her mission to make simulation more realistic for participants. Starting her journey at the University of Delaware, she found the current offerings lacked “the human factor” and was determined to put out superior products that allow learners to actually learn how they will practice. Avkin was established in 2015 and has been selling into the North American markets since 2016. The need for quality healthcare education is a worldwide phenomenon and Avkin is thrilled to announce it is extending its reach into the United Kingdom and Ireland through a strategic distribution partnership with MDT Global Solutions . Cowperthwait and the Avkin team have worked with the team at MDT Global Solutions on a variety of other initiatives, and they are confident this quality partnership and overwhelming enthusiasm for wearable simulators in the UK is a giant leap forward to improving health outcomes globally. Cowperthwait commented, “You want to work with people that have the same passion to improve healthcare education through simulation and have the respect within the global simulation community to share it with the masses.” MDT Global Solutions Ltd was established in 2013 by Rob Clark and David Halliwell. With a background in Pre-Hospital Education that spanned more than 50 years, reinventing simulation became a huge passion. The aim of MDT Global Solutions is to provide innovative solutions to clients. Rob Clark Co-Founder and Director commented, “The team here at MDT admire the ethos, innovation, and passion that Amy and the Avkin Team bring to the Healthcare education market, and are honored to have been selected to become a Distribution Partner here in the United Kingdom and Ireland.” Because of their mutual respect and historical relationship, MDT Global Solutions has been approved to sell Avkin’s entire product line in the UK and Ireland including, but not limited to, Avtrach, a wearable tracheostomy simulator with imbedded sensor technology that will change the way tracheostomy care is taught in the region. Megan Weldon, CHSE, Avkin’s Director of Education, commented “We do everything we can to make our products mimic actual bedside care. The most important thing is that learners can now safely practice realistic patient care before they provide care to a real person.” In addition to Avtrach and six other wearable products, Avkin released an automated, wearable simulator that mimics childbirth. Avbirth allows participants to interact with a real person, practice different birthing positions, and experience all phases of uncomplicated and complicated labor. “This product is a game-changer for nursing schools and midwifery programs” Weldon comments. The Avkin team firmly believes that it is essential to include themes of diversity and marginalization in the simulation setting, especially since this modality gives the patient a voice in the simulation and debriefing process. Because of this, each product is offered in three different skin tones. According to The Guardian , Black women in the UK are four times more likely to die during pregnancy and childbirth. This shocking number has everyone take pause and come together to do their part to improve education and address the disparity of care head-on. Avkin would like to extend our gratitude to MDT for this incredible partnership opportunity. We are confident this partnership will lead to improved educational outcomes that will quickly result in improved care for patients and their families across the UK and Ireland. Both companies are on a mission to create the highest-fidelity simulated learning environments for their customers. By adding a human-to-human aspect to regional simulation centers, the partnership will unlock new possibilities.

This significant announcement means that EOlife is now a fully approved medical device and is immediately available for clinical use in cardiopulmonary & respiratory arrest patients. “The CE mark for EOlife represents 2.5 years of hard work and is a milestone for ARCHEON. Our company was created to develop and market its first device dedicated to the ventilation of patients in cardiac arrest” explains Valentine. “A technical and technological challenge for an entire team: our aim was to offer users a ready to go solution that perfectly meets the identified need. In order to achieve this objective, we had to design a complex product, integrating mechanics, electronics and software, which implies to meet numerous standards and regulatory requirements. Over the last two years, EOlife’s effectiveness has been proved with numerous demonstration and test sessions, as well as its compliance with the safety and performance requirements set out in EU Medical Device Regulation 2017/745. The results obtained now make it possible to award the CE mark to EOlife, the final step before launching it on the European market.” ” EOlife is a system without equivalent or precedent on the market. When we embarked on the path of innovation in the field of medical devices, we knew that the road ahead would not be an easy one. At the same time as developing the product, the company had to set efficient working methods common to all. EOlife has developped a solid and sustainable quality approach, guaranteeing safe products for users. At that time, it was a start from scratch process for a young start-up like ours. In this quest, we looked for subcontractors and suppliers that share the same high standards as we do. This is mandatory to do so, when producing high-performance, high-quality products. We would not have been able to overcome these challenges without a formidable collaborative spirit and the mobilisation of our entire team. Today, we are all very proud having achieved this, and seeing our efforts become reality. We today celebrates the CE marking with all the team. Our ambition now is that EOlife will become an essential tool for ventilation in cardiac arrest. Also we at ARCHEON will continue to innovate in order to improve the care of patients in vital emergency situations. ARCHEON has all the assets to become a recognised company in the field of emergency care. Our company, in only two and half years of existence, has proven its capacity to bring a cutting-edge medical device onto the market, enabling complex problems to be solved. We are convinced that this is just the beginning of a long story.”

In today’s marketplace there is an ever increasing pressure on organisation’s capital budgets, but there is always a need and a desire to replace, upgrade, and improve medical equipment to stay at the forefront of the technology available in order to ensure the best possible patient outcomes. MDT Finance allows you to spread the cost of MDT equipment enabling immediate acquisition, with no capital outlay required. MDT Finance is designed to support your procurement requirements with a straightforward and NHS compliant finance solution. Rental periods can be structured for up to 5 years and for NHS customers we can even defer the first rental payment by up to 12 months, enabling you to get the equipment you need now but with payments commencing in your new financial year. Benefits ■ Enables immediate acquisition of equipment ■ No capital outlay required ■ Spread the cost of equipment for periods up to 5 years ■ Fixed costs for easier budgeting ■ 1st payment can be deferred for up to 12 months ■ Value for money alternative to outright capital purchase End of term options At the expiry of the Fixed Term Rental period there are a number of options: ■ Use of the equipment can continue at a reduced secondary rental, for a period of your choosing ■ The equipment can be returned (subject to return conditions) ■ The equipment may be purchased outright Procurement made easy When discussing your equipment requirements with your MDT Global Solutions product specialist, why not ask for an indicative rental quotation to be included with the equipment quotation. You may be pleasantly surprised by how cost effective the payments are, in addition to the many other benefits it offers. Need more information? We have a dedicated MDT Finance specialist who will be pleased to explain how this offering can work for you. For more information on how we can make it easier than ever to acquire the MDT Global Solutions equipment you need, please contact: Edward Rowe T: 07860 144 647 E: ed@rental-solutions.co.uk
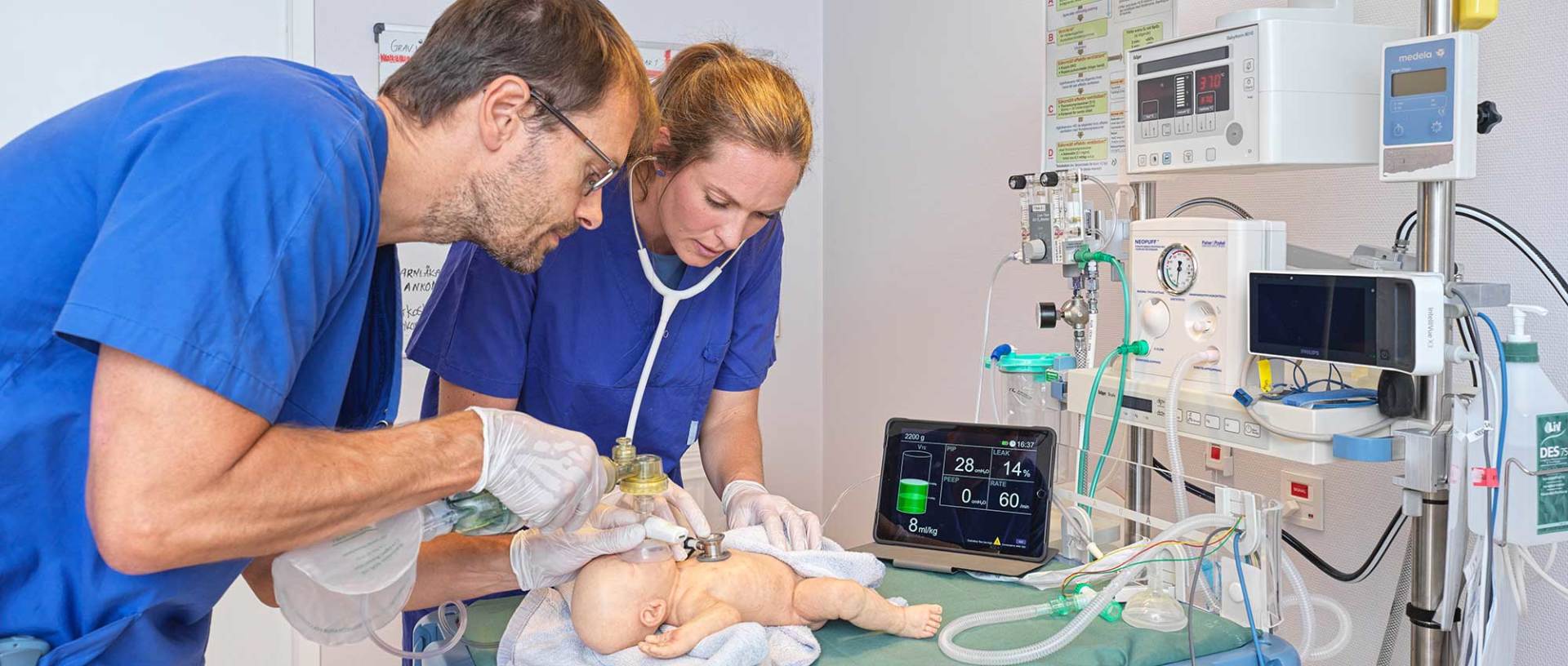
Monivent announce CE marking and delivery of the first units of Neo100 After many years of development work, Monivent have completed and CE-marked Monivent NEO100 and also delivered the first units. It has been a long journey, but through the fantastic work by Monivent's team, they now have a product ready for use on newborns in the delivery room. The EC certificate required for CE marking, which shows that the product meets all applicable requirements for safety and performance in the medical technology regulations, was received at the end of April 2020 after review by the notified body. After completing the production step, Monivent were able to CE mark the NEO100 in mid-July.

MDT Global Solutions Ltd. announced today that they have been appointed as the exclusive distribution partner for the French based manufacturer, Archeon Medical. The agreement covers the distribution of their innovative EOlife® Ventilation monitoring system, designed to provide breath to breath monitoring of ventilations to ensure that safe, effective & consistent volumes are delivered to patients requiring emergency ventilatory support. Dave Halliwell and Rob Clark, Co-Directors / Founders of MDT said; “We are proud to have been selected by Archeon to represent their ground-breaking EOlife product in the UK & Ireland and we look forward to bringing this life saving technology to emergency care providers in both Hospital and Pre-Hospital arenas.” Alban De Luca, CEO & Founder of Archeon said; “We are very excited to sign this distribution agreement with the MDT Team and benefit from their significant background as professional caregivers in the NHS. We are aware that manual ventilation training is a corner stone to improving the management of cardiopulmonary resuscitation. That is an asset to build a strong partnership for the commercial launch of EOlife and to help save many lives.” About Archeon Medical Archeon was set up in January 2018 by Alban De Luca, a biomedical technology engineer and Pierre-Edouard Saillard, an embedded electronic system engineer. Archeon has developed EOlife first device for assisting ventilation during cardiopulmonary resuscitation which is still the leading cause of death worldwide and survival rates remain below 5%. One of the main challenges facing emergency first aid teams is providing patients with enough oxygen, whilst avoiding hyperventilation which can have severe aftereffects. For further information regarding this release, please contact: Stuart Hildage, MDT Global Solutions Ltd. stuart@mdtglobalsolutions.com
